Learning Objectives
- Describe resource, resource partitioning, character displacement, and the niche concept
- Identify factors that define niches, distinguish between fundamental and realized niches, and give an example of this concept
- Explain how competition reduces fitness for both species involved, and characterize the long-term consequences of competition: coexistence, competitive exclusion, resource partitioning, and character displacement
- Recognize and define the following interspecific interactions: competition, enemy-victim interactions, commensalism, amensalism, and mutualism
Resources and Niche Concept
As we consider how organisms interact with organisms of other species in their environment, we need to officially define some ideas that we’ve been bandying around in class. Organisms need many things to survive, including food, water, sunlight if you photosynthesize, etc. Many species need oxygen and carbon dioxide, but there are entire groups of bacteria and archaea that live in anoxic conditions and don’t use or require oxygen. Not all of these required elements are called resources. A resource in ecology is a thing or factor that causes population growth and that is reduced by use. By this definition, oxygen isn’t a resource for humans unless we are living in space or underwater or somewhere requiring we get our oxygen from a finite tank. Likewise, sunlight is not considered a resource for most photosynthesizing organisms. The resource that is reduced the most by use, for example water in a desert, and thereby limits population growth first is specially designated as a “limiting resource.”
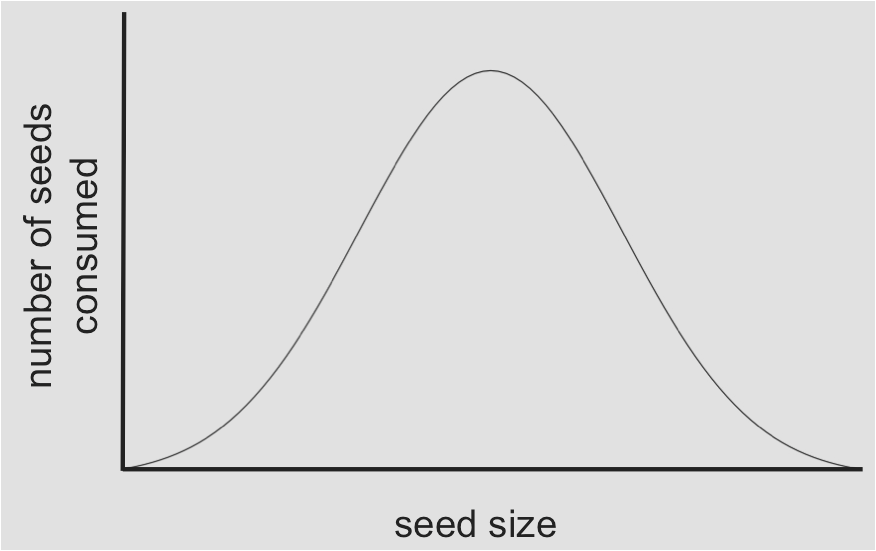
The full range of resources that a species can use combined with the range of conditions a species can tolerate (now we can add sunlight and oxygen back into the mix) defines the ecological niche for that species. Niches have many dimensions, one for each requirement of the species, but we usually reduce a complex niche down to the one or two dimensions deemed most important for the question we are testing. The figure below shows a range of seed sizes to define the niche breadth on the x-axis, with seed consumption on the y-axis.
The figure above shows the fundamental niche, the full resource axis of seed sizes that a finch species is theoretically able to use. Frequently in nature we observe that a species only uses part of its fundamental niche; the part they use is called the realized niche.

- Where is the realized niche for the green species on the left?
- Is the green or purple species the stronger competitor?
Competition between species for resources is strongest where their niches overlap. In the zone of overlap, intense competition can result in the species using a realized niche smaller than its fundamental niche. In communities with many similar species, the result is resource partitioning, where realized niches pack together for several similar species. This is one pathway to coexistence between species as an outcome of competitive interactions.
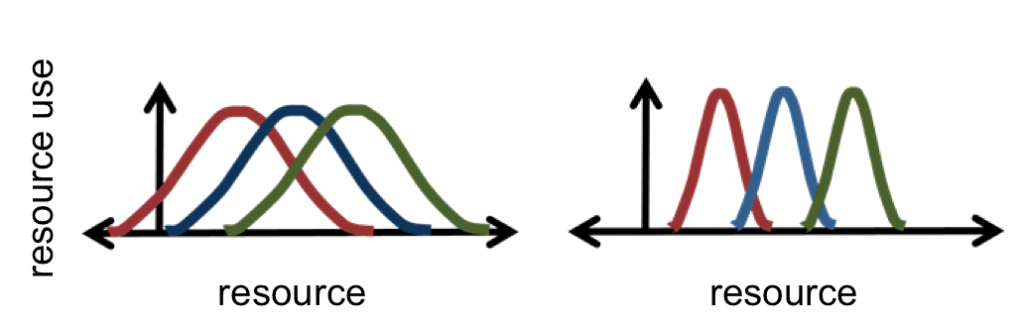
These ecological interactions can exert such strong selection pressure that the trait to use the resource (like the beak of the finch to eat seeds) can adapt over generations to a distinctly new character state (like a smaller or larger beak better matched to the available seed food supply). The shift in the trait value is called character displacement. Character displacement is an evolutionary adaptation in a heritable trait, caused by resource partitioning. If the trait in question lacks enough heritable variation for character displacement to occur, then outcome can instead be competitive exclusion, where the more successful species will out-compete the other to the point of elimination.
Competitive interactions have negative effects on both species
The competition for resources that drives reductions in niche overlap is called interspecific competition, and it has a negative effect on both species in the interaction. That is, individuals of each species would have more success (more resource access with less energy expenditure = greater growth, survival and reproduction) in the absence of the other species. We can consider this mathematically with a slight alteration of the logistic equation to include the detrimental effect of species 2 on species 1, where the two species are represented with subscripts.

The new term in this equation is “minus alpha[12].” Alpha is the competition coefficient, and you can think of it as converting units of species 2 into units of species 1. For more individuals of species 2 or larger values of alpha, species 1 sees lower population growth. You can create the complementary population growth equation for species 2 by swapping the 1s for 2s and vice versa. Competition is a lose-lose interaction. It’s also very very common because resources are finite and necessary for survival and reproduction.
Predation, Parasitism, and Herbivory are detrimental to one species while beneficial to the other
Another class of interspecific interactions has a negative impact on one species while providing benefits to the other: predation, parasitism, and herbivory.
Predation, or predator-prey interactions, involve one species consuming (eating) the other- a clear win-lose scenario. Another win-lose scenario is parasitism, or parasite-host interaction, where a species (the parasite) lives on or in another species (the host) that it harms over time, sometimes resulting in host death. Parasitism is a win for parasite and a loss for the host. Herbivory is similar to predation in the sense that a grazer eats a plant. However, some grazing interactions can be argued to provide a benefit because grazing can promote the growth of new plant material. To determine if an herbivore-plant interaction is a win-lose, the cost and benefit to the plant would need to be measured.
Mutualisms benefit both players in the interspecific interaction
Mutualism is the final class of interactions we will consider by name. In a mutualism, both species benefit from the interaction. For example, plants have fungi that live on or in their roots, called mycorrhizae. The fungus gains access to sugars like glucose and sucrose from the root system, and the plants gain access to the phosphorus and nitrogen that the fungi “recover” from decaying organic matter (which would otherwise be unavailable to the plant). Mutualisms are vulnerable to cheating, where one pair in the interaction saves time or energy and circumvents the benefit to the other species. For example, pollinating insects transfer pollen (sperm) from one plant to another as they forage for nectar in the plant’s flowers. Exact matches between the flower structure and the pollinator’s mouth and tongue structure show evidence of strong co-evolution. However, some insects cheat on this interaction, such as the bumblebee in the images below:


One-way interactions: Commensalism and amensalism (excerpted from OpenStax CNX)
In commensalism and amensalism, one organism gains or loses but the other is unaffected. Commensalism is a relationship where one species benefits from the close, prolonged interaction, while the other is not impacted or affected. For example, when birds nest in trees, the tree is not harmed. Nests are typically light, producing little strain on the branch, and sit below the photosynthesizing leaves. The birds benefit greatly by the height and seclusion of a nest in a tree. Another example of commensalism is the clown fish associating with the sea anemone. The sea anemone is not harmed by the fish, and the fish benefits with protection from predators who would be stung upon nearing the sea anemone.
In amensalism, one organism inflicts harm without receiving any costs or benefits. The bread mold Penicillium produces the compound penicillin. Penicillin is toxic in general to bacteria, including bacteria that would not harm the mold species. On a more macro scale, the movement of large terrestrial vertebrates like elephants crushes grass and small terrestrial invertebrates, with no noticeable effect on the elephant.
Interspecific interactions are not static but can evolve
All biological interactions are dynamic, and interspecific interactions are no exception. Mutualisms, for instance, are susceptible to cheating by one member of the interaction, which can shift the interaction to parasitism. Likewise, a parasitic interaction, such as Wolbachia bacterial infection in Drosophila that reduces female fecundity in the flies, can evolve rapidly from parasitism into mutualism (Weeks et al. 2007).
Here’s Hank Green’s Crash Course review of Community Ecology:
UN Sustainable Development Goal

SDG 2: Zero Hunger – Understanding how organisms interact through competition, predation, parasitism, and herbivory is crucial for sustainable agriculture and food production. By studying the interactions between plants and herbivores, we can develop more effective pest management strategies and reduce the use of harmful pesticides and create more sustainable crop yields for reliable food sources.