Learning Objectives
- Describe the basic procedure for cloning vertebrate animals via somatic cell nuclear transfer to enucleated eggs
- Discuss the difficulties and obstacles, both technical and ethical, for the use of animal cloning
- Compare and contrast therapeutic cloning versus reproductive cloning
- Describe the procedure for obtaining embryonic stem cells
- Compare and contrast embryonic stem cells with alternative stem cell sources (iPSCs and adult stem cells)
Gene therapy works best by genetically repairing a patient’s stem cells. The easiest source of stem cells are from early embryos. The intersection of stem cell technology, genetic engineering, and cloning poses both scientific and ethical challenges. Before we consider how molecular cloning works, watch this video to learn what stem cells are and how they form:
From the video, we now know that stems cells have two key features: differentiation and self-renewal. This can help us understand some strange things about some cell types. Differentiation, the process of a cell type becoming specialized, explains how our different tissues and structures form from a single zygote cell as multicellular organisms develop and grow. Self-renewal means that a cell can replicate itself faithfully. Red blood cells, which do not have nuclei at all, don’t contain the genomic DNA that all other body cells have, and without the genetic code these cells cannot self-renew. Instead, new red blood cells are generated from hematopoietic stem cells found in bone marrow.
Reproductive Cloning
Many organisms, including all bacteria and archaea and some eukaryotes, reproduce asexually. Asexual reproduction results in progeny that are genetically identical to the parent, meaning that they are “clones” of the parent.
Most complex, multicellular eukaryotes, however, reproduce only sexually. Two haploid gametes unite to form a diploid cell, called a zygote, that reproduces mitotically to form all the somatic cells of a complex multicellular organism. During mitotic cell divisions, various cells express different sets of genes to differentiate into different organs, tissues, and cell types. Two fundamental questions of biology are: 1) how do genes regulate the process of development, and 2) do somatic cells undergo irreversible genetic changes as they differentiate.
Early experiments with cloning in plants showed that individual somatic cells (cells that do not form sperm or egg) could form complete, new clonal plants, indicating that the somatic cells had no irreversible changes in their genome compared to the original fertilized egg cell.
The first studies to test whether vertebrate animals could be cloned used a technique called somatic cell nuclear transfer (SCNT), where nuclei from somatic cells were transferred to an egg cell whose own nucleus had been removed.

Early studies with enucleated frog eggs found that donor nuclei from early embryos supported development of a complete adult animal, but nuclei from tadpoles or adult frogs could not. These early results suggested that as vertebrate animals progressed through embryonic development, birth, and aging, their somatic cell nuclei became “programmed” to differentiate into specialized cells, rather than support embryonic development. We now know that this programming involves reversible modification of chromatin that restricts what genes can be expressed in differentiated cells.
The short video below shows the SCNT process:
In 1996, Ian Wilmut and colleagues found that by arresting adult somatic cell cultures in the cell cycle, he could erase some or most of their nuclear programming. Using cultured mammary gland cells from an adult sheep as the source of donor nuclei, he performed 277 SCNTs to create clone embryos. The embryos that divided normally were implanted into the uterus of foster mother sheep. Only a single lamb, Dolly, was successfully born alive and healthy from the 277 attempts. Since then, many other mammalian species have been cloned, with success rates varying from a few to low tens of percent.
This video described how Dolly was cloned as well as other examples of early mammalian cloning experiments: https://www.dnalc.org/view/16992-Cloning-101.html
Mammalian reproductive cloning is still inefficient, with a low success rate, complications during pregnancy, and possible premature aging of the cloned offspring (https://learn.genetics.utah.edu/content/tech/cloning/cloningrisks/). As far as we know, no reproductive cloning of humans has yet been attempted.
Therapeutic Cloning
In contrast to reproductive cloning to create offspring with identical genetic information, therapeutic cloning has a goal to make stem cell lines compatible with the patient to repair a patient’s cells, preferably their stem cells. The stem cell options include adult, modified embryonic, and induced pluripotent stem cells.
Adult Stem Cells
The human body is quite limited in its ability to regenerate or repair injuries or diseases that affect critical organs such as the brain, heart, and pancreas. Tissue and organ regeneration and gene therapy require a source of cells that can differentiate into the desired types of cells and continue to produce those cells for the lifetime of the patient. Adult humans have distinct reservoirs of stem cells, located in different parts of the body (such as the bone marrow). Stem cells, by definition, can continue to divide and both replace themselves and produce progeny cells that differentiate into new cells in their cell lineage, such as blood and immune system cells, or skin cells, or cells that line the gut and airways, or muscle cells. But these adult stem cells are difficult to obtain from a patient, and they are restricted in the types of cells or tissues they can form. For example, the stem cells in the bone marrow can generate both white and red blood cells, but not skin cells or new brain cells or heart muscle or pancreatic beta islet cells (to cure diabetes).
Embryonic stem cells for therapeutic cloning
Cells in an early human embryo, however, are totipotent. Totipotent means they can serve as the basis to form any part of the developing body. Even after the developing zygote differentiates into three germ layers from which all other tissues arise (day 5 in the image below), the three germ layers retain the ability to specialize in different ways. We call these germ layers pluripotent because each layer can become any body cell type within that layer. These pluripotent cells can be cultured indefinitely as embryonic stem cell lines. Existing human embryonic stem cell lines were derived from the blastocysts of early-stage human embryos created through in vitro fertilization in a fertility clinic. The existing human stem cell lines were generated from surplus embryos from fertility clinics that would have been discarded or put into indefinite cryostorage rather than implanted into a human uterus. Without implantation in a uterus, mammalian embryos cannot develop.
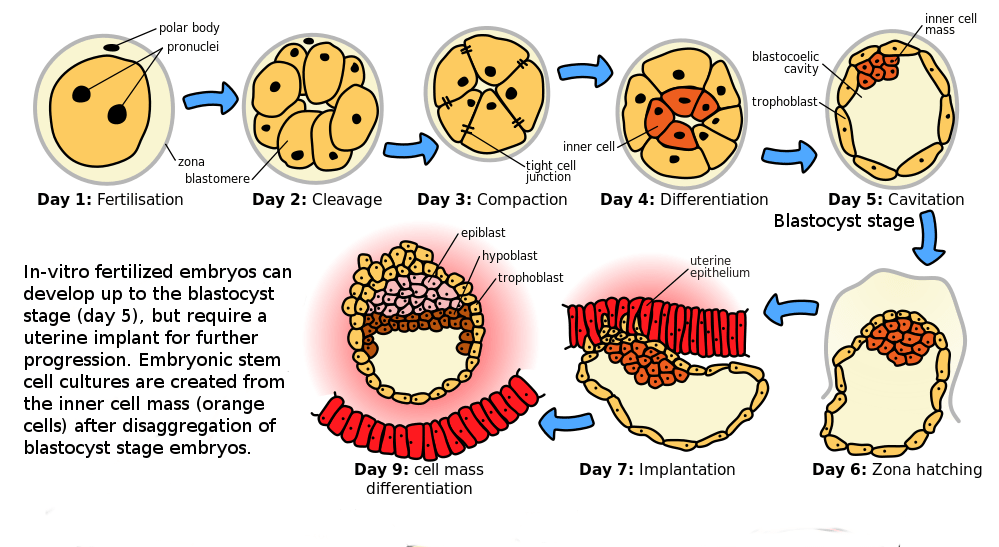
Therapeutic cloning uses enucleated human eggs and somatic cell nuclear transfer technology to create a human embryo that is a genetic clone of the patient. The embryo is destroyed to obtain embryonic stem cells that have the same genotype as the patient. These cells can be cultured indefinitely, and when needed the cells can be hormonally induced to form new tissues and organs that will not be rejected by the patient’s immune system. Human therapeutic cloning – making human embryonic stem cells via somatic cell nuclear transfer – was published for the first time by Tachibana and colleagues in 2013 (https://doi.org/10.1016/j.cell.2013.05.006).
Induced pluripotent stem cells
Beginning in 2006, genetic engineers developed the technology to create a new type of stem cell: induced pluripotent stem cells (iPSCs). iPSCs, created by transforming adult differentiated cells (such as fibroblasts or skin cells) with 4-6 different transcription factors that regulate early embryonic cell growth and differentiation, have many of the properties of embryonic stem cells. The question is whether these transcription factor genes can be safely used to transform the patient’s own cells without causing unacceptably high risks of cancer once these cells are reintroduced into the patient’s body. Because iPSCs do not involve destruction of human embryos, they have been the focus of intense research. Should you be interested in learning more, a review by Wilson and Wu (2015) provides a concise description of the state of the research and the challenges in this field.
Stem cell therapy
Stem cells, depending on whether they were obtained from adults, embryos, or induced with transcription factors, can be induced to differentiate into different cell types to generate replacement organs and repair damaged heart muscle, pancreatic beta cells, spinal cord or brain cells. Coupled with genome editing, stem cells could be used to treat patients with genetic disorders.
Cloning and bioethics
The technical obstacles to reproductive and therapeutic cloning will be addressed and overcome as scientific technologies advance. For instance, the advent of CRISPR, discussed in the previous reading, has quickly revolutionized options to create recombinant organisms. However, in 2016 many of the researchers who discovered CRISPR technology called for a moratorium on use of CRISPR for editing human germ cells and embryos. Consideration of ethical barriers is fundamental to regulate how we use technologies and discoveries. Ideas of autonomy, consent, and individual rights craft the ways that bioethicists and others think about topics like human cloning and “designer babies.” Some ethicists argue that the moral stance and place of humans need also be considered (Sandel 2005) when considering human cloning or the use of embryos in research because humans gain access to the ability to change nature through these technologies. Reflecting as individuals and as societies on the ethics of biology and biological technologies is essential work as cloning techniques advance.
Resources and References
Slides for the videos above: Cloning_StemCells
Wilson, KD and JC Wu (2015) Induced Pluripotent Stem Cells, JAMA. 313(16):1613-1614. doi:10.1001/jama.2015.1846
https://learn.genetics.utah.edu/content/tech/cloning/whatiscloning
https://learn.genetics.utah.edu/content/tech/cloning/clickandclone/ go through the steps to clone a mouse using somatic cell nuclear transfer technology
Sustainable Development Goal

SDG 1: No Poverty – Healthcare plays a role in reducing extreme economic inequalities between and within nations. As new therapies and technologies, such as cloning, become more common, it is necessary to consider the difficulties and obstacles, both technical and ethical, for the use of cloning for healthcare and in increasing food supplies. Who can afford it? By using this technology, what changes take place economically, and for whom? Access to the resources produced by such technologies includes considerations on how to responsibly use biotechnology while minimizing negative impacts on the environment and society across the globe.


Human therapeutic cloning – making human embryonic stem cells via somatic cell nuclear transfer – was published for the first time in 2013:
http://www.npr.org/sections/health-shots/2013/05/15/183916891/scientists-clone-human-embryos-to-make-stem-cells